Comprehensive Dementia Care at Ace: Integrating Diagnosis, Treatment, Research, and Training with COMFORTage for Personalized Patient Outcomes
Introduction to Ace Alzheimer Center Barcelona and its role in the COMFORTage Project
Ace Alzheimer Center Barcelona provides a comprehensive model of dementia care, integrating clinical services, research, and social support. Its Memory Unit and Day Care Unit offer personalized cognitive and functional interventions that enhance quality of life. Within the COMFORTage project, Ace leverages its expertise in early detection and its advanced biobank and clinical database to contribute to the development of predictive AI models and dynamic prognostic criteria. This approach integrates multidimensional data from clinical, neuropsychological and biological data, enabling a personalized, data-driven approach to monitoring disease progression.
COMFORTage allows Ace to implement digital cognitive and functional stimulation strategies that are adaptive and systematically monitored, enhancing therapeutic precision and patient engagement. This collaboration enables AI-enhanced decision-making and supports innovative therapeutic approaches that are dynamically adjusted to each patient’s needs.
By participating in COMFORTage, Ace Alzheimer Center Barcelona strengthens its diagnostic and therapeutic capabilities, advances precision medicine, and supports international collaboration. These positions Ace to contribute to personalized dementia care through AI-driven insights and adaptive digital interventions.
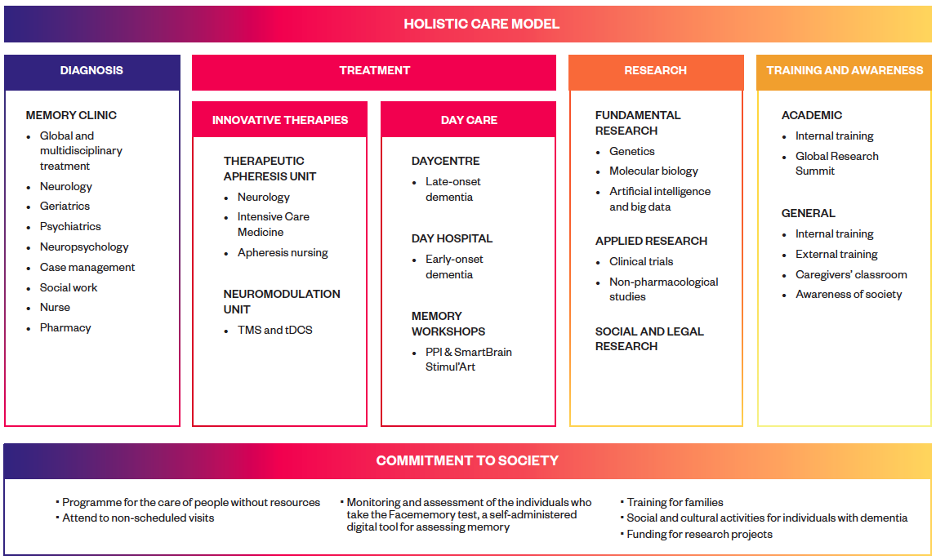
Clinical activities at Ace
At Ace, most patients are referred by their primary care physicians or through a partnership with the public healthcare system, ensuring broad access to specialized dementia care. Each year, the institution evaluates approximately 2,000 new cases and provides clinical follow-up for more than 6,000 patients. All patients undergo comprehensive annual assessments, including neurological, neuropsychological, genetic evaluations, and biomarker analysis, with additional brain imaging (MRI), cerebrospinal fluid (CSF) biomarker analysis, and genomic profiling performed when required. This approach facilitates the early identification of cognitive decline and Alzheimer’s disease biomarkers, ensuring timely intervention. In addition to diagnosing Mild Cognitive Impairment (MCI) and dementia, Ace also evaluates individuals with subjective memory complaints, allowing for the detection of subtle cognitive changes before significant impairment occurs. These individuals are encouraged to participate in observational studies on preclinical and prodromal dementia. All our patients receive annual follow-ups to monitor disease progression, optimize treatment, and evaluate eligibility for clinical trials. Ace follows a holistic intervention model that integrates diagnosis, treatment, research, and training to provide comprehensive dementia care.


Who’s Involved? Participant Recruitment, digital platform, and Data Collection
The Ace pilot study is a randomized, open-label trial designed to compare outcomes between an intervention group and a control group over a two-year follow-up period. The study will include 100 participants aged 60 to 85, divided into two groups of 50 individuals each. Eligible participants will be diagnosed with Mild Cognitive Impairment (MCI) or mild Alzheimer’s dementia.
Participants will follow a standardized protocol, with a consensus diagnosis established by the research team based on neurological, neuropsychological, social, genetic, CSF, plasma, and MRI assessments. These comprehensive evaluations will enable researchers to gain a deeper understanding of the participants’ cognitive profiles and the factors influencing their condition.
The study design consists of two groups:
- Active group (n = 50): Participants will engage in COMFORTage stimulation activities for the first year, followed by no stimulation in the second year. This allows researchers to assess both the immediate and lasting effects of the intervention.
- Control group (n = 50): Participants will not receive stimulation activities and will instead follow general health guidelines and recommendations, serving as a comparison group to evaluate the intervention’s impact.
This structured, randomized design ensures that participants receive consistent evaluations while allowing the research team to systematically assess the impact of digital stimulation interventions on cognitive and functional outcomes over the two-year study period. The study will systematically collect clinical, neuropsychological, and biological data to evaluate participants’ progress and the intervention’s impact.
The Ace study integrates multiple digital tools, specifically designed and customized within the framework of the project, to support cognitive stimulation and data collection. Healthentia facilitates telemonitoring and decision-making through physiological and patient-reported data. Eligence provides interactive brain training for cognitive rehabilitation, allowing personalized exercises tailored to individual needs. Language Games assess cognitive-linguistic abilities through realistic tasks that engage various cognitive functions. Additionally, PUNTO HEALTH evaluates spontaneous speech, analyzing acoustic, lexical, semantic, syntactical, and task-specific features every 3–4 months via a mobile app, ensuring remote and convenient assessment in participants’ homes. This structured multimodal approach enhances monitoring accuracy, allowing professionals to track cognitive performance over time. The data generated will be securely shared within the consortium to refine and validate intervention models.
Face-to-face assessments will be conducted at three key time points: baseline (pre-intervention), year 1, and year 2, providing insights into cognitive and functional changes over time. Self-reported data from standardized questionnaires will be gathered during regular visits, monitoring other objectives of the study. Biological data will include genetic, CSF, and MRI baseline collections, while plasma samples will be taken at baseline, year 1, and year 2 to explore correlations between biomarker changes and cognitive decline.
Why COMFORTage Matters to Ace
The COMFORTage project introduces dynamic prognostic criteria powered by AI-driven predictive models, integrating multidimensional data such as neuropsychological scores, genetic profiles, plasma biomarkers, MRI metrics, and speech analysis. This transformative approach enhances clinical decision-making, enabling personalized, data-driven monitoring of disease progression. A key innovation is the integration of Digital Twins, virtual patient replicas that continuously update with individual data streams. This facilitates predictive simulations and personalized care pathways, allowing clinicians to anticipate disease progression and tailor interventions with precision. The Memory Unit transitions from static diagnostic criteria to a personalized monitoring approach, identifying high-risk patients earlier and optimizing intervention timing and therapeutic strategies. The use of Digital Twins enhances predictive accuracy, supporting proactive and individualized disease management. In the Day Care Units, COMFORTage supports the implementation of digital stimulation strategies within a systematic and adaptive framework. AI-driven insights combined with clinical expertise enable individualized therapeutic processes, ensuring cognitive stimulation programs are dynamically adjusted in real-time to each participant’s needs. For research, COMFORTage integrates clinical, biological, neuroimaging, and digital biomarkers to create comprehensive patient profiles, advancing the development of multimodal biomarkers and deepening the understanding of disease mechanisms. The use of Digital Twins enhances AI model predictive power, enabling longitudinal simulations that inform therapeutic decisions and optimize long-term patient outcomes, while facilitating the creation of unique patient subgroups for targeted clinical trials.

Looking Ahead: The Future with COMFORTage
By participating in COMFORTage, Ace’s Memory Unit and Day Care Units will enhance their diagnostic, prognostic, and therapeutic capabilities while advancing the integration of AI-powered precision medicine and digital therapeutics, contributing to a new standard for personalized dementia care. This involvement places Ace at the forefront of innovative care models, where AI-enhanced decision-making, adaptive digital stimulation, and human expertise seamlessly combine to create a new paradigm of individualized therapeutic care for dementia patients.

